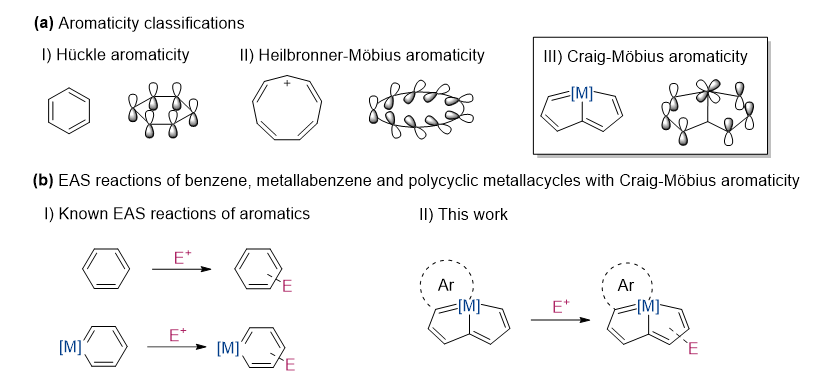
Electrophilic aromatic substitution (EAS) reactions are hallmarks of aromaticity. However, the potential of compounds with Möbius aromaticity to undergo EAS has been ignored for a long time. A number of molecules with d orbitals involved in conjugation of π-aromatic ring in the out-of-phase way have been described as Craig-Möbius aromaticity because they have the same phase inversion properties as Möbius type and fit [4n] rule. Embedding metals within a π-conjugated hydrocarbon skeleton is considered an efficient method for discovering Craig-Möbius aromatic scaffolds. We directly employed metalla-aromatics to develop EAS reactions of Craig-Möbius aromatics with excellent efficiency and remarkable regioselectivity, and the reactions were quantified in computational studies to further rationalize the preferred sites of attack on the different aromatic rings.
URL link for Article: https://www.pnas.org/content/118/39/e2102310118