As we all known, carbon is the most fundamental element in chemistry. “Carbon” also is the protagonist in Organic Chemistry. However, in Coordination Chemistry, the coordinating atoms of polydentate complexes are mainly occupied by donor heteroatoms, such as Nitrogen, Phosphine, Oxygen and Sulfur. Examples of polydentate chelates with exclusively carbon as binding atoms are rare. In the past few years, we discovered that “Carbon” can also be useful chelating atoms. A series of new aromatic frameworks were developed by the combination of carbon-chains with transition metals. For the extensible carbon ligand platform, all of the binding atoms are carbon, we denoted “Carbolong Chemistry”.
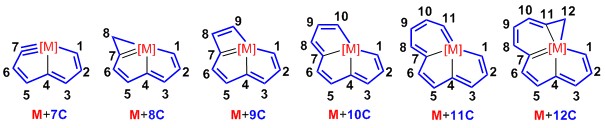

In 2013, Xia, Zhu and co-workers reported the first metallapentalyne(M+7C), which was formed by the interaction of an unsaturated carbon chain with a transition metal. In this tridentate chelate, all of the binding atoms are carbons. Thereafter, with the extension of this carbon chain (ranging from seven atoms to twelve atoms), a series carbon-based polydentate chelates were synthesized. Notably, the CCCCC pentadentate chelates, formed by the coordination of a 12-atom carbon chain to a transition metal, represent the highest carbon coordination number for a metal atom in a planar geometry. This result extends our perception of the chelating ability of carbon-chain. Thus, the concept of the pincer complex can be extended from tridentate to planar polydentate chelates.
The combination of facile synthesis and high stability of these species could make polydentate carbon-chain a novel building block in coordination chemistry. Furthermore, because of their broad absorption from ultraviolet-visible to the near-infrared region and significant photothermal properties, these novel carbon-based polydentate chelates provide a promising material for biomedicine and solar energy utilization.
The development of Carbolong Chemistry
7C carbolong complex: Osmapentalyne
In 2009, the first carbolong complex was synthesized by reaction of the complex 1 with one equivalent alkyne at RT for 5 min. This complex can be viewed as a chelate with carbon tridentate ligand containing 7 carbon atoms. So we call it 7C carbolong complex. As indicated by X-ray structural and magnetic analyses, the bond between the osmium and carbon atom is a triple bond. This is the first example of metal-carbon triple bond within five-membered ring. For the organic analogues, the bicyclic compound, pentalene, is a typical anti-aromatic compound, which is very unstable. In contrast, osmapentalyne shows highly stability even when heated at 120 °C in air.
Chameleonic Reactivities of the Carbyne Carbon
Luckily, we capture the intermediate, metallapentalene 4 in the reaction of osmapentalyne with acid, which has 16 electrons metal center. In this reaction, the carbyne carbon of metallapentalyne shows nucleophilic. It is interesting that this carbyne carbon can also react with nucleophiles, leading to the formation of 18-electron metallapentalenes. Just like a chameleon, the metallapentalyne is nucleophilic when it meets with electrophiles, but shows eletrophilicity when it meets with nucleophiles.
8C carbolong complex: 7+1=8
For the 8C carbolong complex, we attempted to introduce one new carbon atom to the framework of 7C carbolong complex. As shown in the slide, we reacted metallapentalynes with one equivalent of isocyanide reagent to generate 8C carbolong complex 2 in high yield. The further addition of two equivalent isocyanides led to the formation of the first metallaindene, which is also a 8C carbolong complex.
8C carbolong complex: 5+3=8
The 8C carbolong complex can also be synthesized by introducing 3 carbon atoms to the framework of 5C carbolong complex. Reactions of complex 1 with allenes can produce 8C carbolong complexes, which contain three-membered metallacyclopropene units.
9C carbolong complex: 7+2=9
Representing the first [2+2] cycloaddition reactions of alkynes with a late transition metal carbyne complex. The 9C carbolong complex contains both metallacyclobutadiene unit and metallapentalene unit. Here, the two classical unstable antiaromatic compounds, i.e. cyclobutadiene and pentalene, could be stabilized by the replacement of metal fragment. Experimental observations and theoretical calculations reveal that the metal fragment decreases the antiaromaticity in cyclobutadiene and pentalene simultaneously, leading to air- and moisture-stable products. This work was selected as the cover paper of Angew. Chem. journal.
10C carbolong complex: 8+2=10
Actually, 10C carbolong complexes can be obtained easily from the reactions of 8C carbolong complex with one equivalent of alkynes. Due to the large ring strain, the three-membered metallacyclopropene unit of 8C carbolong complex was expanded in the reactions, to form a new five-membered metallapentadiene unit. The compound, which contains three fused five-membered carbocycle are nonplanar and unstable, due to the sp3-hybridized carbon center. In contrast, when the metal serves as the common vertex for the three metallacycles, the fused rings can be planar and stable. Our 10C carbolong complexes are stable up to 200 °C in air.
11C carbolong complex: 7+2+2=11
In the search for the 11C carbolong complexes, we achieved the [2+2+2] cycloaddition reactions of alkynes with carbyne atoms of 7C carbolong complexes. The 11C carbolong complex with coordinated cyclopentadienyl was obtained directly from the reaction of alkyne with electron-withdrawing group. When the alkyne with the electron-donating group, ethoxyl, was allowed to react with 7C carbolong complex, an unprecedented six-membered ring fused intermediate was captured. In this complex, the ring carbon atoms C1 to C8 are co-planar, whereas the other three carbon atoms are distorted from the plane.
12C carbolong complex: 8+2+2=12
We really achieved the 12C carbolong complex recently. By the addition of two equivalent of alkynes or allenes, the 8C carbolong complex can be converted to form 12C carbolong complexes. These complexes are the first examples of CCCCC pentadentate chelates, in which all of the five coordinated carbons lie in the equatorial plane. These structures represent the highest carbon coordination number for a metal atom in a planar geometry.

One-pot synthesis of carbolong complexes
In addition to the step-by-step methods mentioned above, recently, we established a one-pot method based on multiyne chains (we termed them carbolongs) for the construction of carbolong complexes. This work represents the first example of direct bonding a single organic molecule and a metal entity to construct conjugated polydentate chelates with more than two metal-carbon σ bonds, motivating the re-recognition of the carbon coordination ability as σ-donating binding atom.

Carbolong complexes with a ruthenium center
The metal centers of carbolong complexes are capable of extending to other transition metals. A series of such species with ruthenium were obtained by carbolongs chelating commercially available OsCl2(PPh3)3. Notably, ruthenapentalyne I is the first cyclic metal carbyne complex with a second-row transition metal center. DFT calculations suggest that the inherent aromatic nature of ruthenapentalyne I enhances its stability. Reactivity studies showed striking observations, such as ambiphilic reactivity, metal-carbon triple bond shifts, [2+2] cycloaddition reactions with alkynes and cascade cyclization reactions with ambident nucleophiles.
A number of carbolong complexes containing hetero-atoms: the chelating atoms are still carbons
Properties and applications
Photoluminescent Properties of Osmapentalynes
In contrast to organic aromatics, the fluorescence of osmapentalyne exhibits large stokes shifts, Near-Infrared emission and long lifetime. Most fluorescent aromatics are planar molecules and their emission is usually quenched in their aggregates due to π–π stacking interactions. Such an ‘aggregation quenching’ phenomenon is generally considered to be disadvantageous in applications. But, in sharp difference, osmapentalyne show the aggregation-induced emission enhancement, because π–π stacking of the fluorophores is hindered sterically by the bulky ligands.
UV-Visible-Near Infrared Absorption Spectra
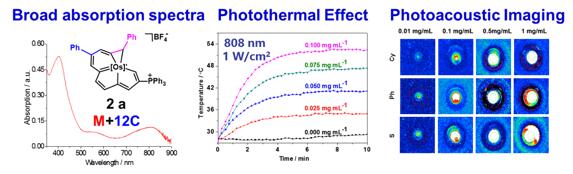
The absorption spectra of these carbolong complexes are also very interesting. Upon increasing the size of the aromatic framework, the absorption maximum in the visible region is red-shift gradually. For the 12C carbolong complex, the absorption spectra almost cover the entire visible region and extend to the near infrared region.
Especially, the absorption spectra of carbolong complexes can be tunable by introducing different substituents to the rings. For example, the absorption maximum of the 7C carbolong complex is located at 694 nm, and its molar extinction coefficient is even larger than the values of the typical dyes, such as N3, N719, and black dye. In addition, the broad absorption of the 8C carbolong complex can nearly reach 1200 nm.
Photoacoustic Imaging(PAI) in vivo
One important function of theranostic nanoplatform is to image the delivery process and obtain the information about drug accumulation in disease region. To get such information, in vivo PA imaging was performed on SCC7 tumor bearing mice. PA imaging could confirm the carbolong complex accumulation in tumors area.
Photothermal therapy
The broad absorption spectra in the NIR region make the 12C carbolong complex promising candidate for photothermal therapy. As shown in the Figure, the temperature of the water–ethanol solution containing the 12C carbolong complex significantly increased from 28 to 52 °C within 5 min. In addition, the photoacoustic signal increased with the increasing concentration of carbolong complex as indicated by the photoacoustic imaging detected through photoacoustic instrument. This is the first example of organometallic complex as photothermal and photoacoustic conversion agent.

The stabilizing influence of the aromaticity was quite evident, in that the combination of the carbolongs with different metal centers to form the carbolong complexes with notable stability. To gain more insight into the compatibility with other transition metals, the NICS evaluations of the parent models with metal centers from groups IVB, VB, VIB, and VIIB were computed. All the calculated NICS values turned out to be negative. The survey raises the intriguing possibility of a future synthesis of stable carbolong complexes with early-transition metal centres. The carbolong chemistry is on the way.




















